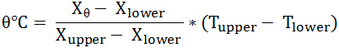|
|
ThermometerThermometerTo measure temperature accurately, we have to use a thermometer, which makes use of thermometric substances.
Thermometric substances: substances (solid, liquid or gas) that have physical properties that vary continuously and linearly with temperature.
Features of a good thermometer
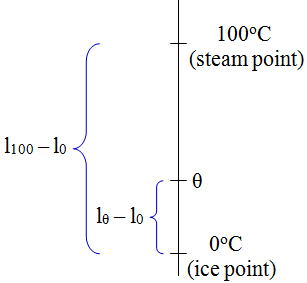
Calibration of ThermometerStep 1: Determine two fixed points on an unmarked mercury thermometer
Definition
Step 2: Divide the interval between ice point and steam point into a 100 equal parts. Each interval is 1⁰C. Temperature of unknown substance
Method 1: Once the Celsius scale is determined from above, we can simply place the calibrated thermometer into the unknown substance and get the reading. Method 2: After getting l0 and l100, we can again find the height of the mercury level lθ when the thermometer is placed in the unknown substance. Then the temperature of unknown substance is found using this equation θoC = (lθ - l0)⁄(l100 - l0) X 100oC Note:
This equation can be applied in any thermometric properties discussed previously. where X = any thermometric properties
upper = upper fixed point lower = lower fixed point T = temperature Note that Tupper - Tlower is not always 100⁰C. Example
A thermocouple indicates 0.1 mV at ice point and 2.6 mV at 500⁰C. What will be the temperature when the thermocouple indicates 3.0 mV? temp = (3 - 0.1) ⁄(2.6 - 0.1) X (500 - 0)= 580oC |
|