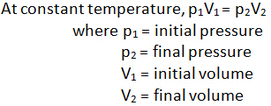|
|
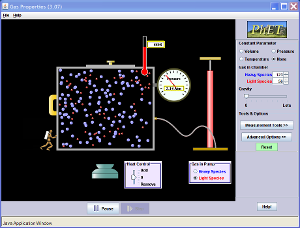
Pressure in GasesHow does a gas exert pressure on the walls of its container?
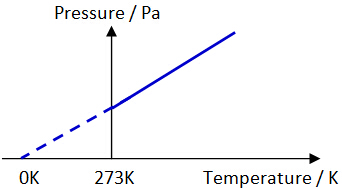
Air molecules are in constant random motion. They collide the walls of the container and exert a force on it. Since pressure is force per unit area, pressure is created on the walls. Pressure-Temperature (p-T) relationship of a gas
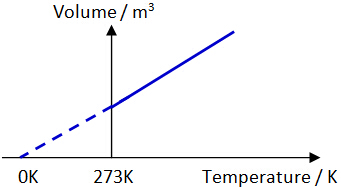
Volume-Temperature (V-T) relationship of a gas
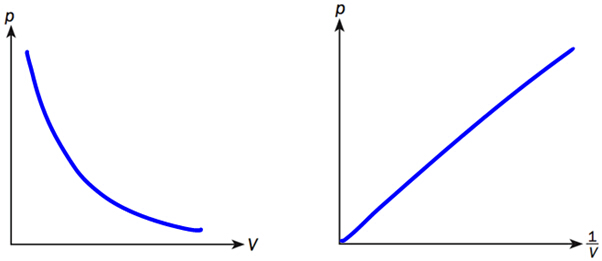
Pressure-Volume (p-V) relationship of a gas
In general, pressure, volume and temperature of a fixed mass of gas can be related by this equation.
pV = nRT where p = pressure V = volume n = number of mole (constant as mass is fixed) R is a constant T = temperature Note that this equation is not in O-level syllabus, but you can use it to analyze the relationships between pressure, volume and temperature. To help you understand these concepts better, you can play with the applet below.
(d) Steps for Volume-Temperature Relationship
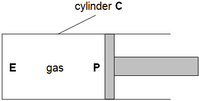
(a) Use the kinetic molecular model of matter to explain why the pressure on P is equal to that on the end E of the cylinder.
(b) While keeping the temperature constant, the piston is now moved to the left such that volume of the gas is reduced by one-tenth. Calculate the final pressure of the gas. (c) What happen when the gas is heated. Answer: (a) The gas molecules hit the piston as well as all the walls of the container equally. Equal number of molecules will hit the surface of the piston as well as end of the container, E. The frequency of collision is the same thus, pressure is the same. (b) P1V1 = P2V2 1.0 x 10^(5) x V1 = P2 x 0.9 V1 P2 = 1.11 x 10^(5) Pa (c) When the gas is heated, it gains kinetic energy and moves faster. They collide more frequently and forcefully with the wall of cylinder, which then increases the pressure inside the cylinder. As this pressure exceeds atmospheric pressure, the piston P is then pushed to the right. |
|