|
|
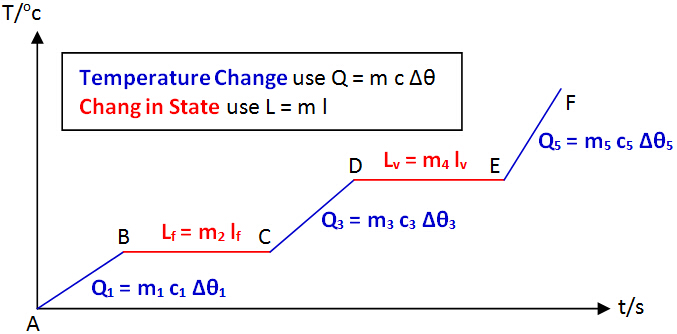
Heat CalculationSteps to do a calculation in thermal physics
Step 1: Determine which material releases thermal energy (temperature decreases) and which one gains thermal energy (temperature increases) Step 2: Determine whether the substance undergoes any change of state. Step 3: Apply the formula and solve the problem by making an assumption that no energy is lost to surrounding unless question stating otherwise. The following heating curve will help you in determining which formula to use. Example: A piece of hot coal of mass 50 g at a temperature of 200oC is dropped into 10 g of ice at a temperature of -10oC. Calculate the final temperature reached. (The specific heat capacity of coal, ice and water are 710 Jkg-1K-1, 2100 Jkg-1K-1 and 4200 Jkg-1K-1 respectively. The specific latent heat of fusion of ice is 336000 Jkg-1) Analysis: Working: Recall that change in temperature in ⁰C is same as change in temperature in K. Therefore, you do not need to convert the unit for temperature change. This is discussed under Kelvin Scale.
|
|