|
|
Brownian MotionParticles are too small to be seen by our naked eye or even powerful microscope, and Brownian motion provides a clear evidence for kinetic model of matter.
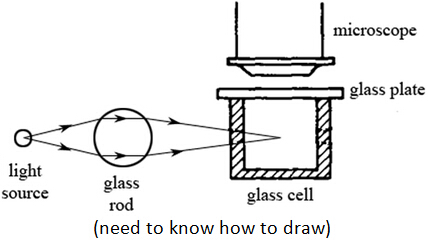
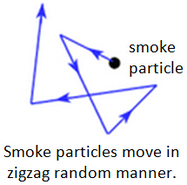
Brownian motion is the constant random motion of tiny particles suspended in a fluid (i.e. smoke particles in air or pollen grains in water). Brownian motion is caused by the bombardment of air molecules on the smoke particles in air or water molecules on the pollen grains in water. ExperimentObservation: Bright spots which are the smoke particles that reflect lights are seen to move at constant random motion.
Explanation: The air molecules collides the smoke particles from different directions and at different times. Due to uneven collisions, the smoke particles are at random motion.
Conclusion: The air molecules are moving at constant random motion at high speeds. A Brownian Motion simulation can be found here. Note
|
|